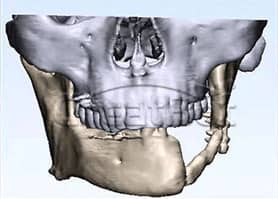
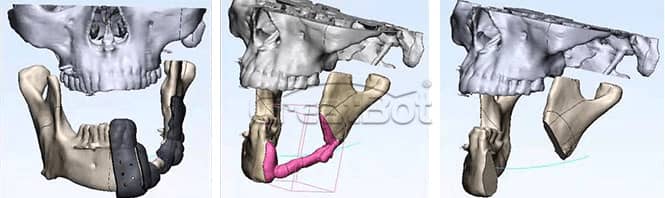
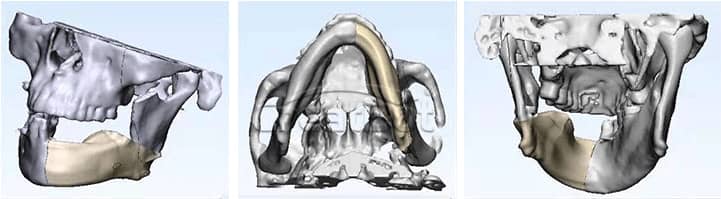
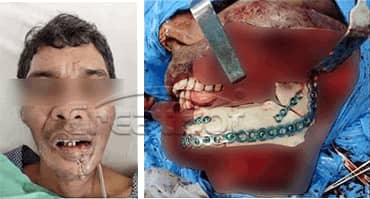
CreatBot medical grade PEEK hard tissue regereration (HTR) prosthesis for cranioplasty and osteogenesis are authenticated by FDA, USP, ISO, etc, which has good biocompatibility, wear resistance and stable chemical properties, can be disinfected with high temperature steam or gamma radiation. Since the first cranial implantation in 2016, there have been no negative effects such as rejection and infection. Up to now (August 2019), more than 50 cases of medical cases have been realized, and more than 20 cases of PEEK implants have been achieved, all of which have performed well and are favored by various international medical groups. Many thanks to professors from Johns Hopkins University (the highest institution in the world of medical science), who provide unparalleled technical support and case feedback to us with CreatBot 3d printers. Now more and more international medical teams join the CreatBot 3D printing PEEK solution.
Contact us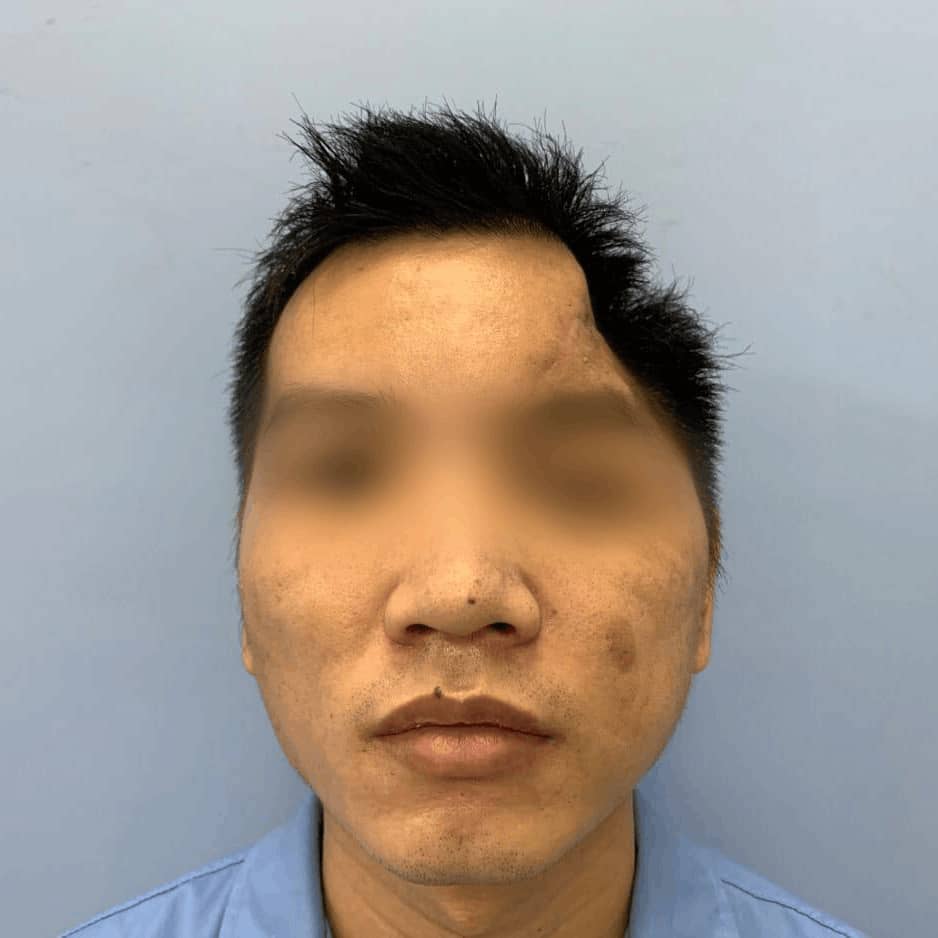
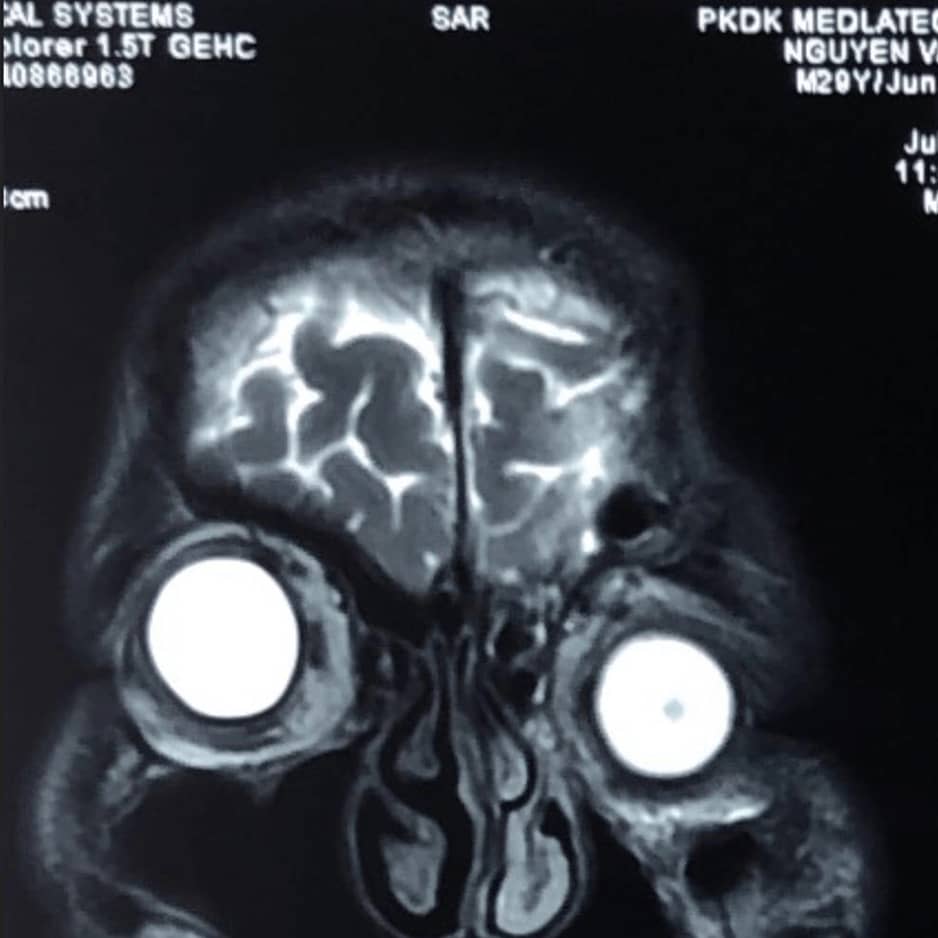
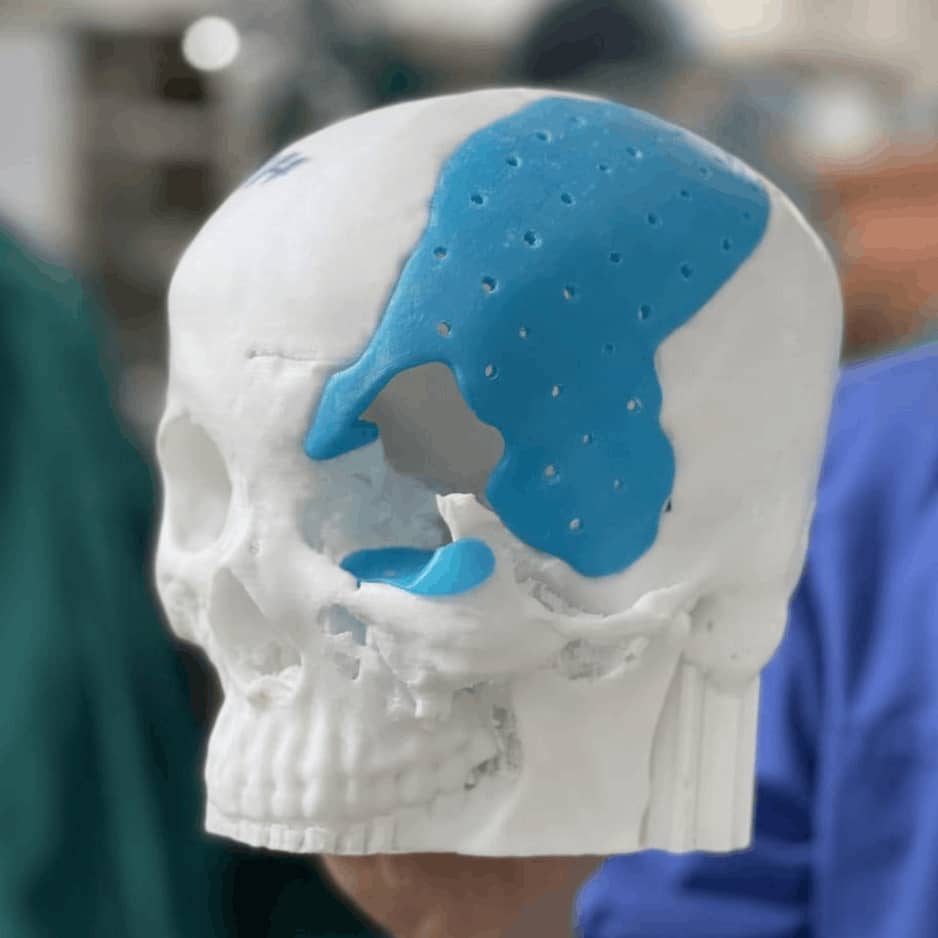
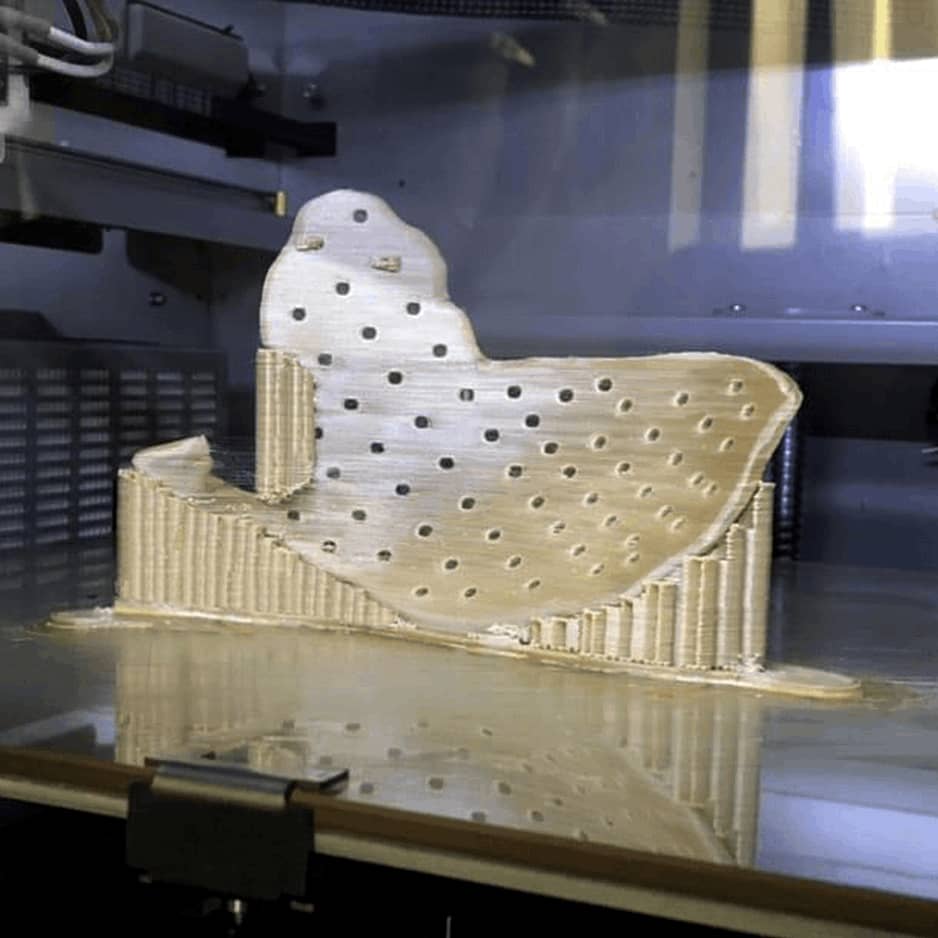
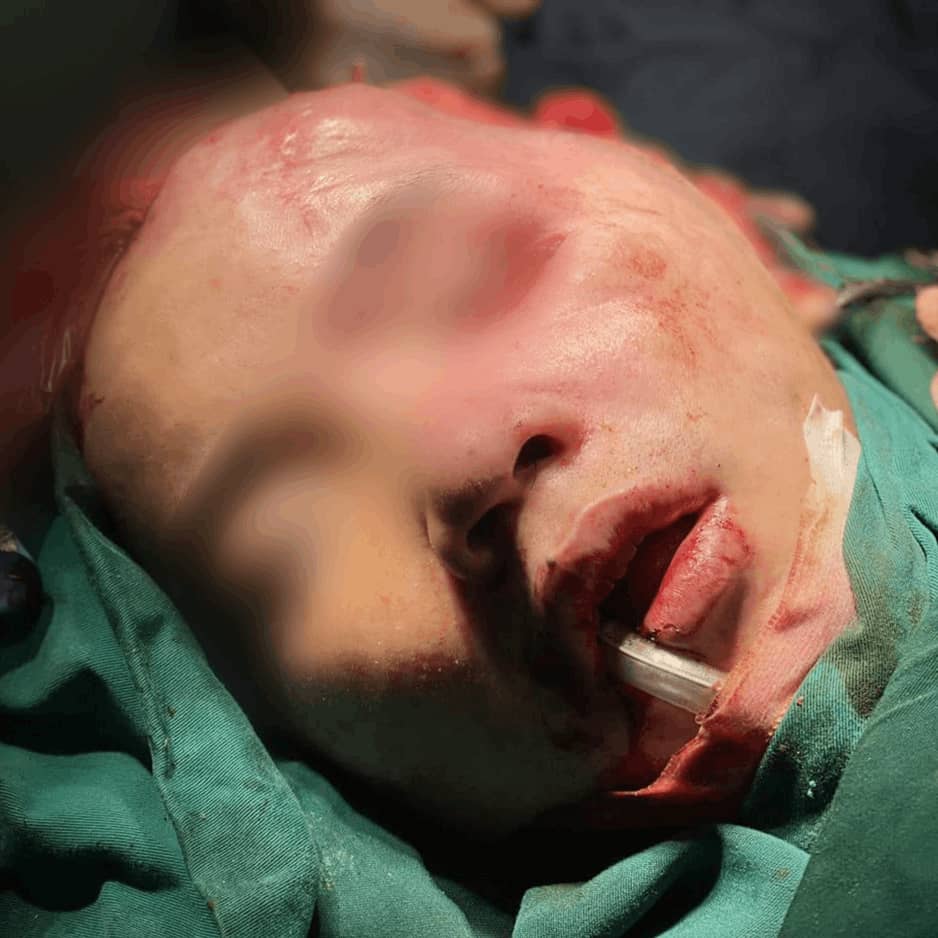
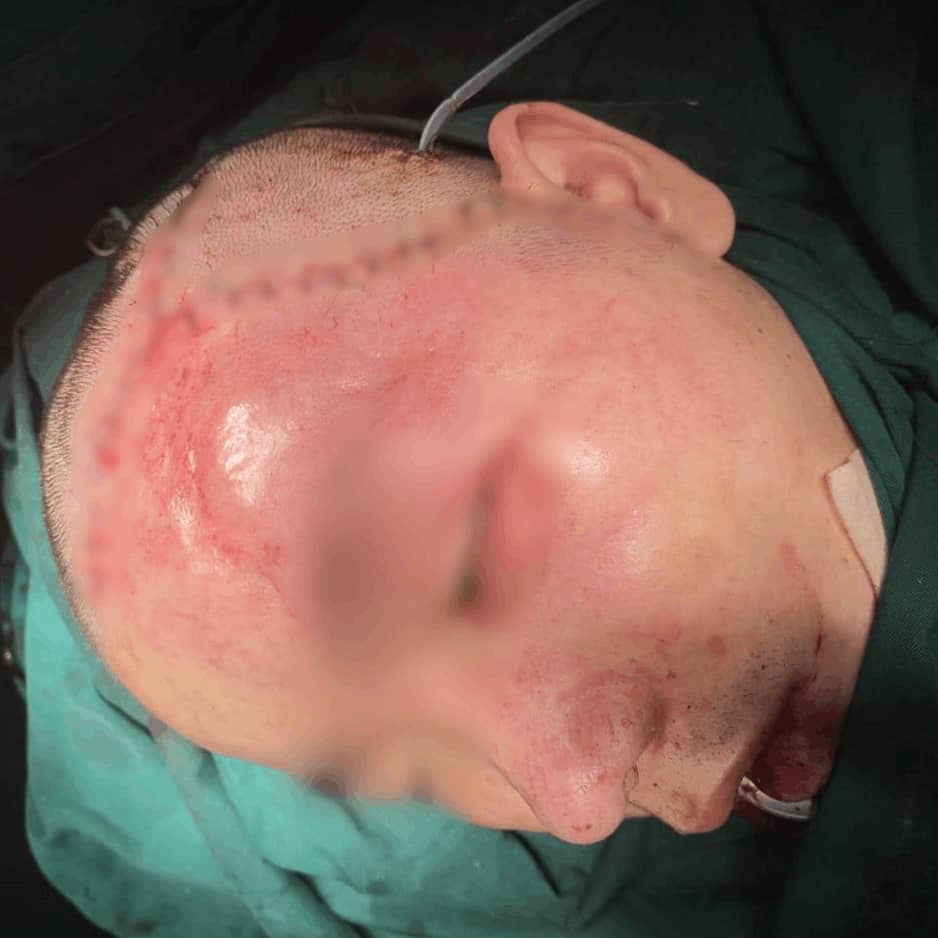

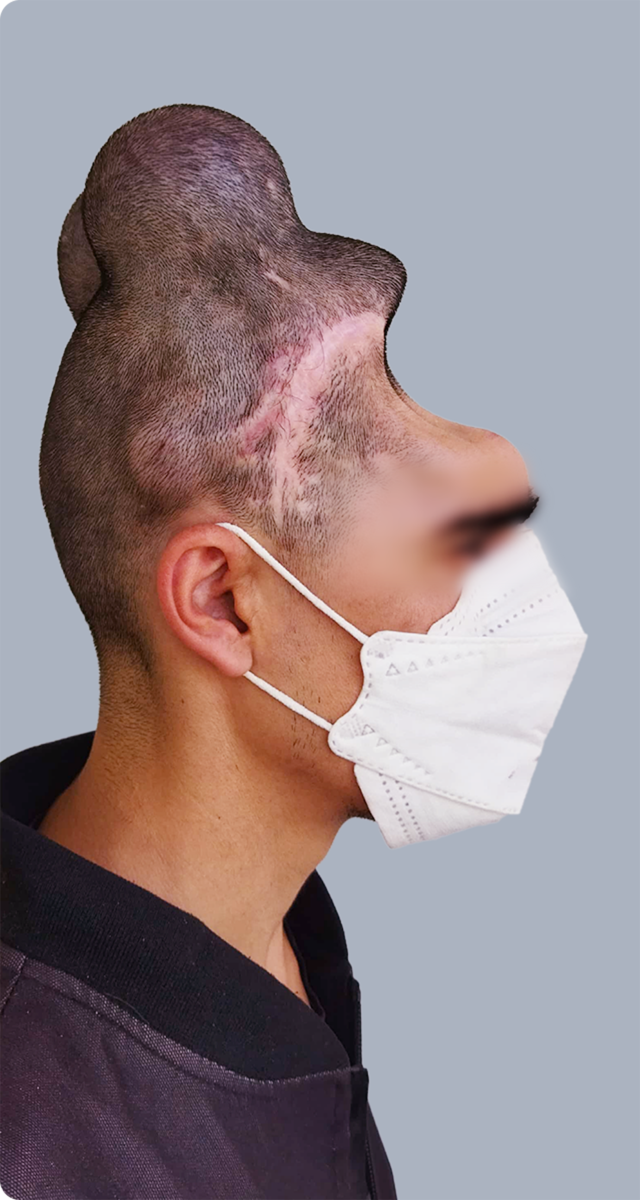
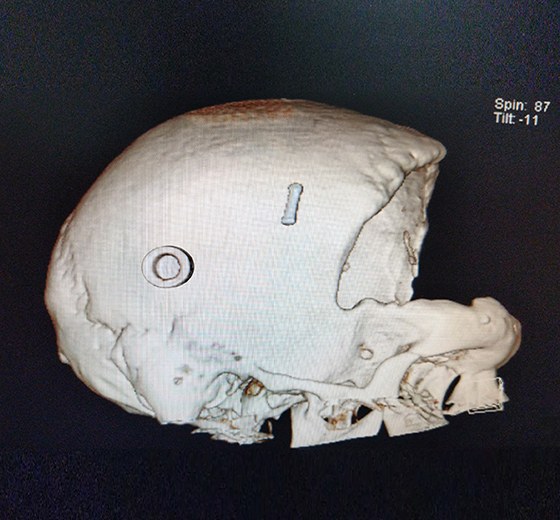

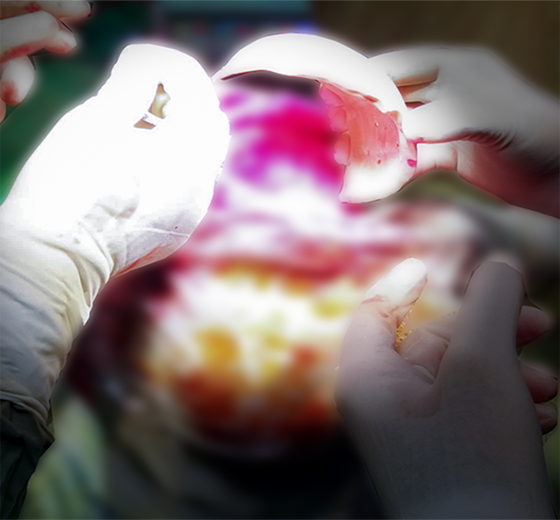

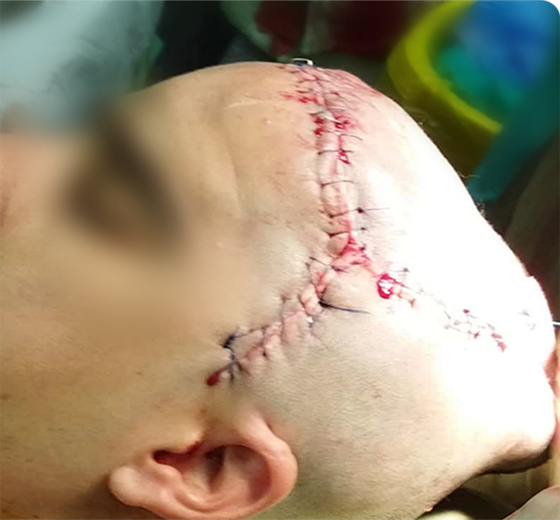
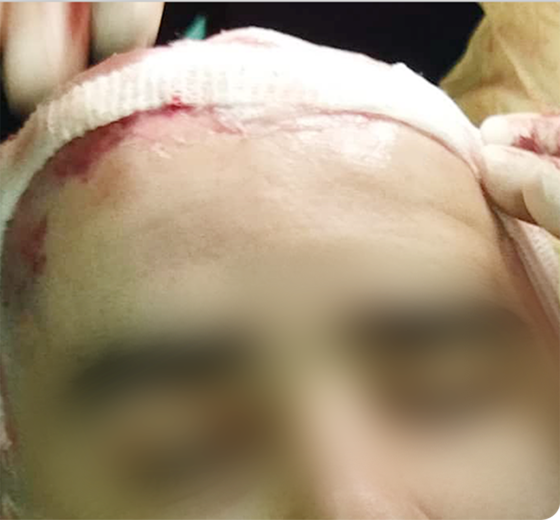
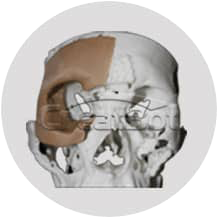
Sample Verification
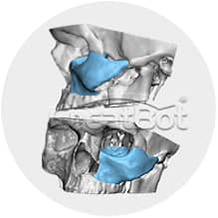
Design Model

Model Sliced

3D Printing Process
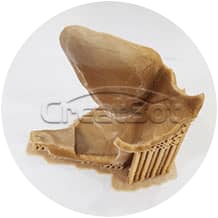
Before Annaling
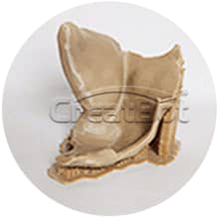
After Disinfection And Annaling
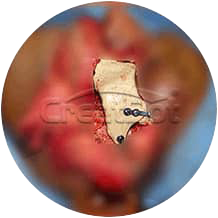
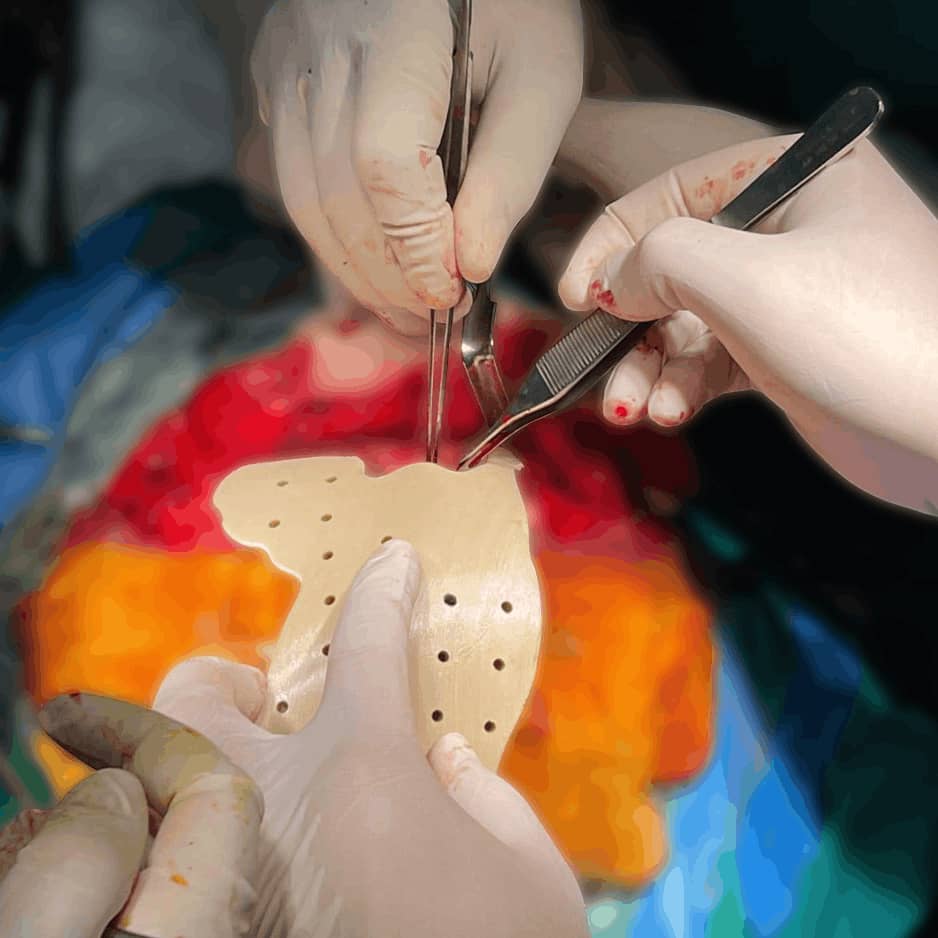
Implant Body
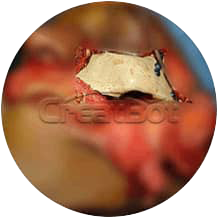
Implant Body


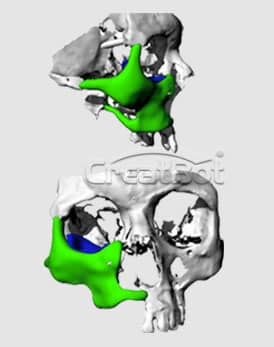

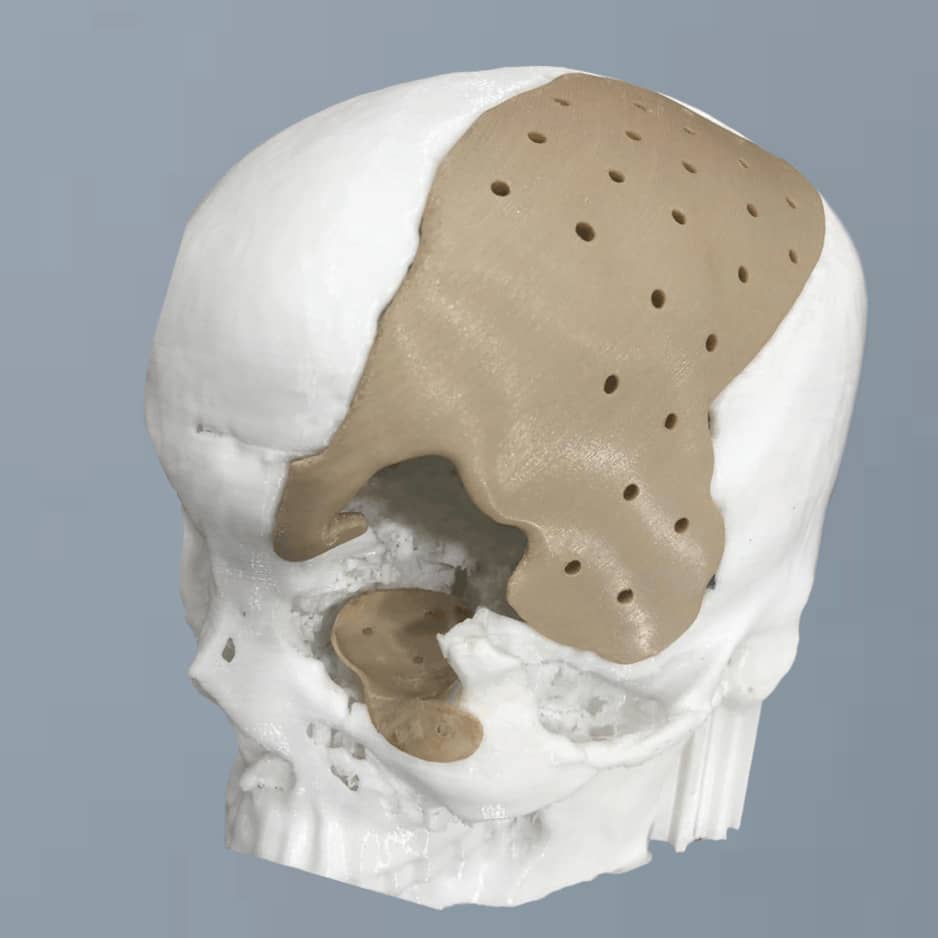
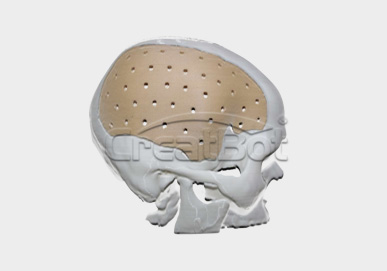
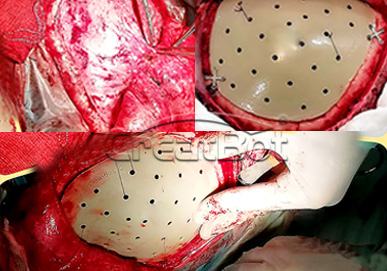
PEEK Cranium Implant





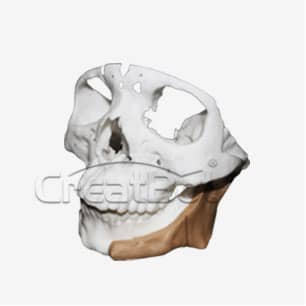
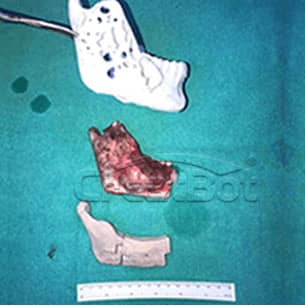

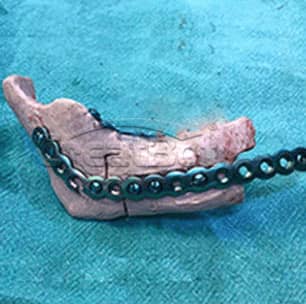
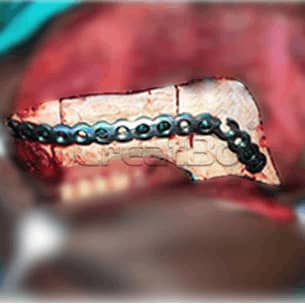
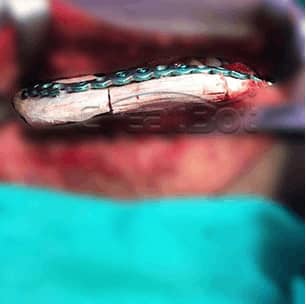
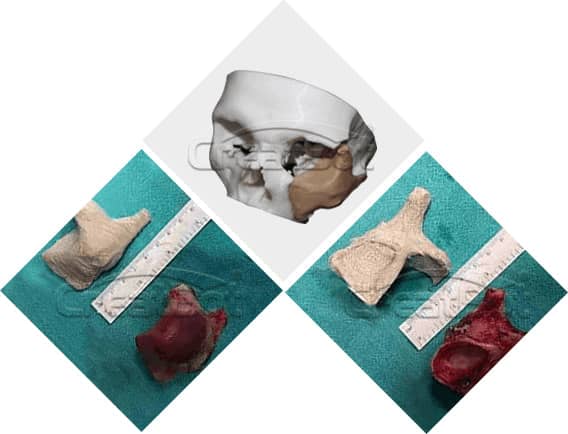
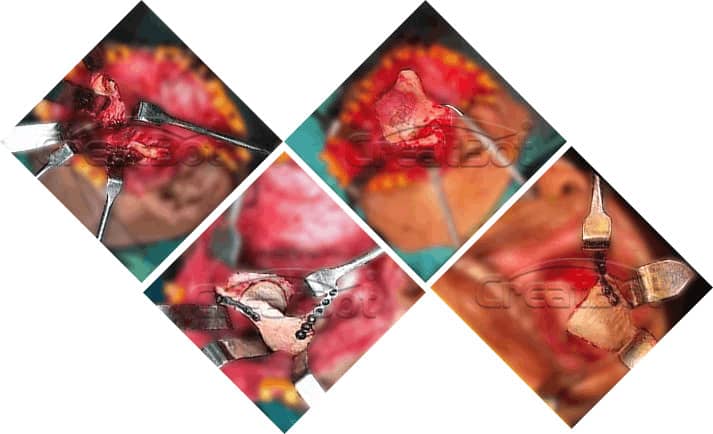

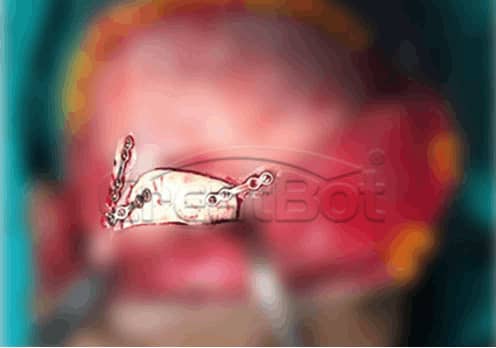
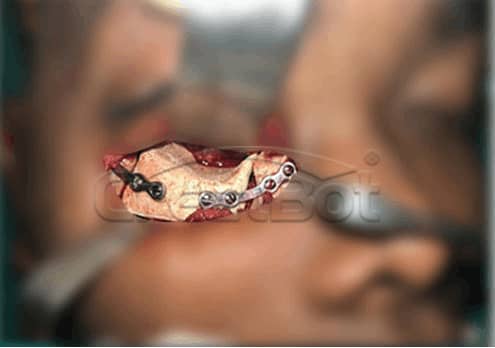

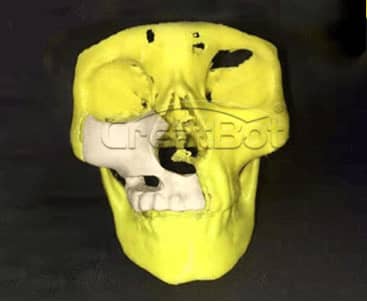

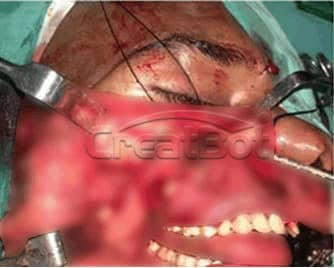
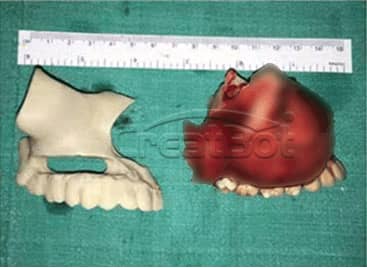
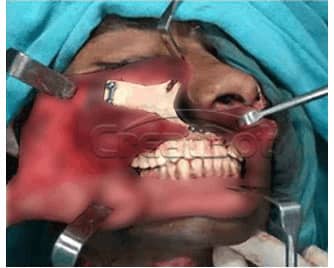
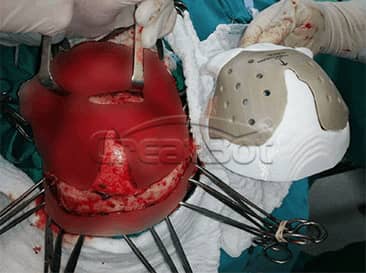
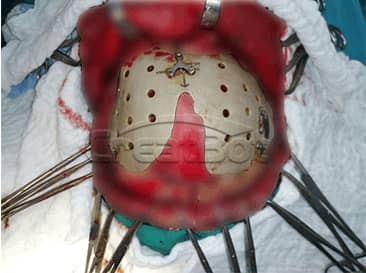

lower Jawbone PEEK Implant
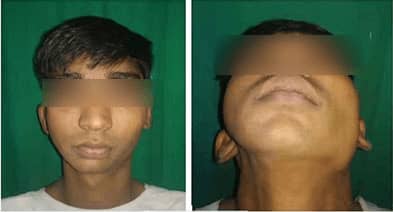


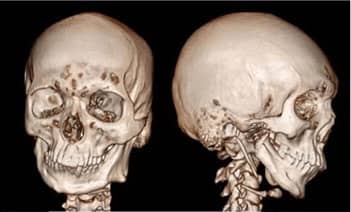


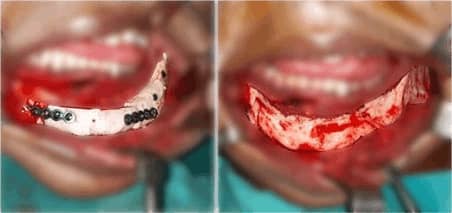


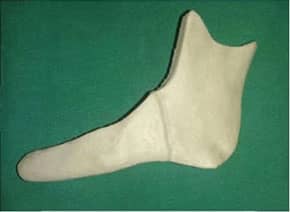






lower jawbone PEEK implant